Abstract
Background: MF is a hematologic malignancy characterized by splenomegaly and debilitating systemic symptoms. The Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF) questionnaire was developed for patient self-report of MF symptoms (Emanuel 2012). Although there have been several versions of this questionnaire, they have demonstrated consistent reliability, cross-sectional validity, and high level of comparability for both TSS and individual symptoms (Dueck 2017). The JAK2/FLT3 inhibitor PAC has demonstrated durable spleen volume reduction and symptom control in patients with MF irrespective of baseline platelet count in 2 phase 3 trials vs BAT (Mesa 2017, Mascarenhas 2018). Since changes in study design and external factors limited the ability to assess TSS in PERSIST-1 and PERSIST-2, respectively, a pooled analysis of all available TSS data from both phase 3 trials was undertaken to further evaluate TSS response.
Methods: PERSIST-1 was a phase 3 study of PAC 400 mg QD vs BAT (excluding ruxolitinib) in patients with MF (excluding those with prior ruxolitinib treatment); PERSIST-2 was a phase 3 study of PAC 400 mg QD or 200 mg BID vs BAT (including ruxolitinib) in patients with MF and baseline thrombocytopenia (platelet count ≤100x109/L); 48% of patients in PERSIST-2 had prior ruxolitinib and 44% of patients randomized to BAT received ruxolitinib treatment during the study. In PERSIST-1, 2 versions of the questionnaire were administered; MPN-SAF TSS in the first 179 patients and MPN-SAF TSS 2.0 in the subsequent 148 patients. In PERSIST-2, all 311 patients used the MPN-SAF TSS 2.0 questionnaire. In both trials, the efficacy endpoint associated with TSS was defined as the proportion of patients achieving ≥50% reduction in TSS from baseline to Week 24 based on MPN-SAF TSS 2.0. In the post-hoc analyses of PAC vs BAT herein, data were presented based on the pooled MPN-SAF TSS 2.0 responses, as well as the pooled MPN-SAF TSS and MPN-SAF TSS 2.0 (6 symptoms in common) responses from PERSIST-1 and PERSIST-2. A multivariate stepwise analysis was also performed using potential prognostic factors for MF as predictors of TSS response. These analyses were performed in the intent-to-treat efficacy populations in both studies: all patients randomized in PERSIST-1 and all patients with randomization date allowing for Week 24 data in PERSIST-2.
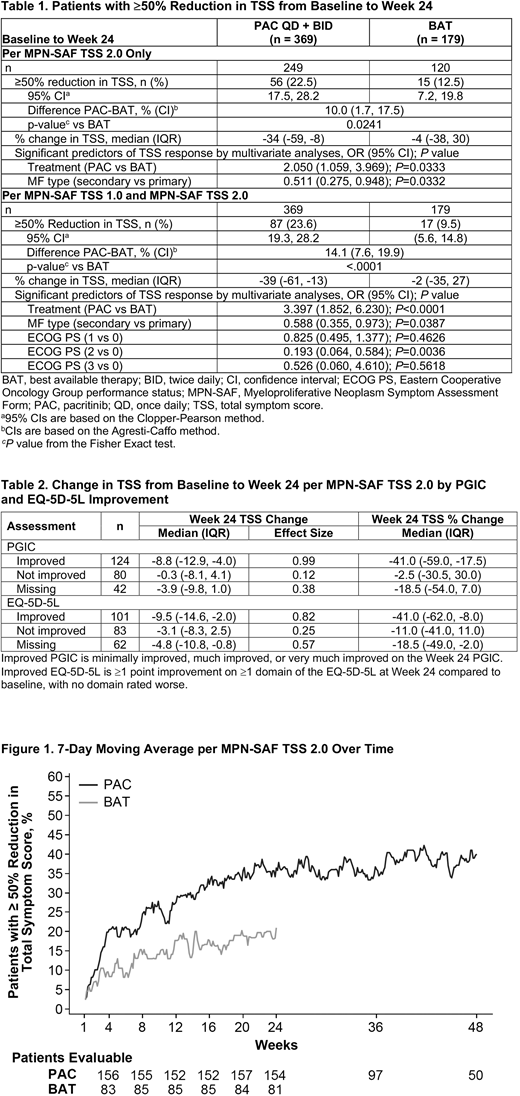
Results: Data from 548 patients (n = 369 PAC, n = 179 BAT) were analyzed, with 179 patients administered the MPN-SAF TSS and 369 administered the MPN-SAF TSS 2.0. Baseline patient and disease characteristics were balanced for PAC and BAT groups, with a higher rate of primary MF in the PAC group (66% vs 57%). Median TSS scores at baseline were 19.5 (range, 0.3-57.3) and 19.6 (range, 1.6-55.9) in PAC and BAT groups, respectively. Results of the pooled analysis demonstrate significantly higher rates of ≥50% reduction in TSS from baseline to Week 24 with PAC vs BAT for MPN-SAF TSS 2.0 (P=0.0241) and the pooled versions of the questionnaire (MPN-SAF TSS or TSS 2.0; P<0.0001; Table). In multivariate logistic regression analyses of treatment group and baseline potential prognostic variables, only treatment group (PAC vs BAT) and MF type (secondary vs primary) were significant predictors of TSS response for MPN-SAF TSS 2.0 and for the pooled versions of the questionnaire (Table 1). Study (PERSIST-1 vs PERSIST-2) did not significantly impact TSS response (P=0.2243), supporting the validity of data pooling. Reduction in symptoms with PAC were rapid, occurring as early as Week 4 (Figure). Patients in the PAC group achieved a steady state response rate of ≈35% around Week 20, while steady state response rate in the BAT group was ≈15% around Week 12. Additionally, larger absolute reductions and percent reductions in TSS at Week 24 were correlated with improvement as measured by Patient Global Impression of Change and change in EQ-5D-5L (Table 2).
Conclusions: The integrated results from the phase 3 PERSIST-1 and PERSIST-2 trials demonstrate significant improvement in the rate of TSS response with PAC vs BAT. Overall, phase 3 data demonstrate that PAC was significantly more effective than BAT for the reduction spleen volume and symptom burden in patients with MF irrespective of baseline platelet count or prior treatment with JAK2 inhibitors.
Mesa:Pfizer: Research Funding; Novartis: Consultancy; Incyte Corporation: Research Funding; Promedior: Research Funding; Gilead: Research Funding; NS Pharma: Research Funding; Celgene: Research Funding; UT Health San Antonio - Mays Cancer Center: Employment; CTI Biopharma: Research Funding; Genentech: Research Funding. Cervantes:Celgene: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Hospital Clinic Barcelona: Employment. Harrison:Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau; Roche: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Speakers Bureau; CTI BioPharma: Consultancy, Honoraria; Gilead: Honoraria, Speakers Bureau. Mead:Bristol-Myers Squibb: Consultancy; Celgene: Research Funding; ARIAD: Consultancy; Cell Therapeutics: Consultancy; Elstar: Research Funding; Evotek: Research Funding; Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau. Verstovsek:Incyte: Consultancy; Celgene: Membership on an entity's Board of Directors or advisory committees; Italfarmaco: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Wang:CTI BioPharma: Employment, Equity Ownership. Granston:CTI BioPharma: Employment, Equity Ownership. Mascarenhas:Merck: Research Funding; Novartis: Research Funding; CTI Biopharma: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Research Funding; Promedior: Research Funding; Janssen: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal